
Overview of the JW and JZ Modifier
A growing number of prescription drugs, including some of the most expensive ones, are dispensed in variable doses that are administered based on a patient’s weight or body surface area (BSA) rather than in fixed or flat doses. Prescription drugs such as antibiotics, Accutane, and many other medications are taken in pill or tablet form. By contrast, many biologic/cancer drugs are administered by weight. Biologics for treating Atopic Dermatitis, Psoriasis, Psoriatic Arthritis, Eczema, Melanoma, Basal and Squamous Cell Carcinomas have greatly increased treatment options for the more severe cases of these conditions.
Such medications have no such thing as a standard dose. Instead, each patient receives a personalized dose based on his or her weight or body size. Single-dose vials come only in a limited number of specific sizes, so the amount of the drug contained within a vial often exceeds the required weight-based dosage for a given patient, and whatever amount is left over must be discarded.
In 2017 Medicare added Modifier JW with instructions to append it to claims billed for biologics that are paid under Medicare part B with units for unused and discarded amounts from single-dose containers or single use packages. Instructions are included as to how to document in the chart the amount of drug that was discarded.
A new ruling, effective July 1, 2023, instructs providers and suppliers to report modifier JZ on all claims that bill for single-dose containers of drugs that are payable under Part B when there are no discarded amounts (when all the drug or product is used).
Modifier | Short Descriptor | Long Descriptor |
JW | Discarded drug/product not administered | Drug/product amount discarded/not administered to any patient |
| JZ | Zero drug/product wasted | Zero drug/product amount discarded/ not administered to any patient |
Both JW and JZ should only be used for claims for single-dose container drugs. The JW and JZ modifier policy applies to all providers and suppliers who buy and bill separately payable drugs under Medicare Part B.
In general, the JW and JZ modifier policy applies to all drugs separately payable under Medicare Part B that are described as being supplied in a “single-dose” container or “single-use” package based on FDA-approved labeling. The use of these modifiers is not appropriate for drugs that are from multiple-dose containers.
Even if a drug is excluded from the definition of “refundable single-dose container or single-use package drug” (and not subject to the discarded drug refund), for example, multiple source drugs, claims for such drugs furnished from a single-dose container are still required to use the JW and JZ modifiers. The JW and JZ modifiers are not required for vaccines described under section 1861(s) (10) of the Act that are furnished from single-dose containers.
Providers and suppliers may report the JZ modifier prior to July 1, 2023. It is available for use beginning January 1, 2023.
How to bill with JW and JZ
Using a single-dose container that is labeled to contain 100 units of a drug to administer 95 units to the patient and 5 units are discarded. The 95-unit dose is billed on one line, while the discarded five units must be billed on another line with the JW modifier. Both line items would be processed for payment. If no drug or product is wasted, one claim line must include the billing and payment code (J Code), NDC # and units administered or used with modifier JZ. Utilizing the JW and JZ modifiers assures compliance with the guidelines as well as providers being reimbursed for all the medication in the vial.
The following is for example purposes only.
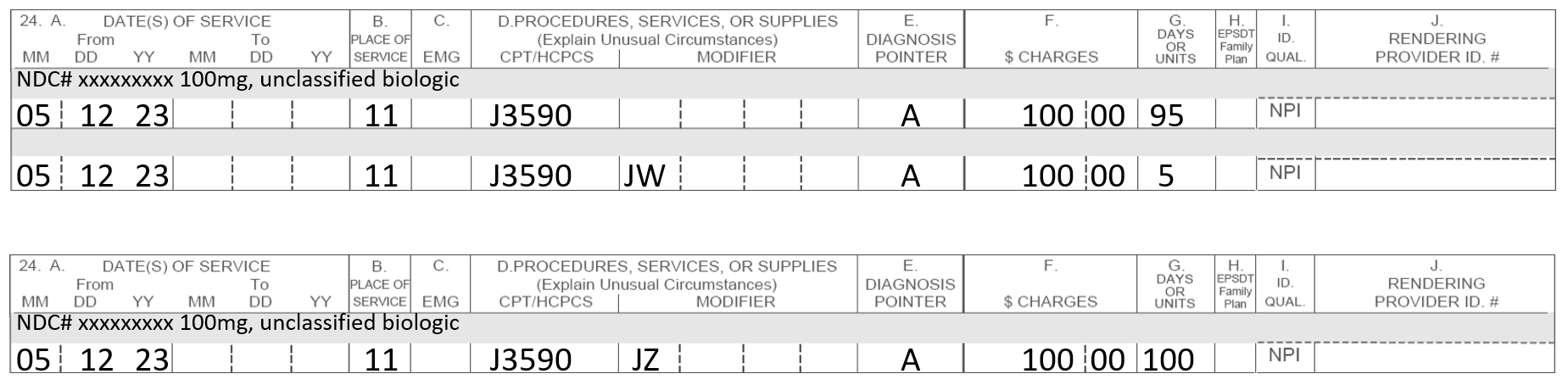
Note: The NDC# for the biologic is listed above the date, POS, etc.
Claims for drugs/biologics should be billed using the HCPCS/CPT code that most accurately describes the drug. Drug administration services provided in physicians’ offices should be billed as follows:
96372 | Therapeutic, prophylactic, or diagnostic injection (specify substance or drug); subcutaneous or intramuscular. |
However, other third-party payors may allow the use of CPT code 96401 – Chemotherapy administration, subcutaneous or intramuscular, non-hormonal, antineoplastic. According to the American Medical Association’s CPT codebook, CPT code 96401 applies to substances including certain monoclonal antibody agents, as well as other biologic response modifiers.
What are the billing requirements for the JW Modifier?
CPT codebook states that “the highly complex infusion of chemotherapy or other drug or biologic agents requires physician or other qualified health care professional work and/or clinical staff monitoring well beyond that of therapeutic drug agents (96360-96379) because the incidence of severe adverse patient reactions are typically greater.” Coverage and billing requirements for this code vary by payer, so providers should consult with the non-Medicare payer to determine whether, and under what circumstances, this code may be used.
If a significant and separately identifiable Evaluation and Management (E&M) service is performed on the same day, the appropriate non-facility-based E&M CPT codes (e.g., 99202-99205, 99212-99215) should be reported with modifier -25 in addition to CPT codes 96372 or 96401. A different diagnosis is not required for an E&M service provided on the same day. CPT code 99211 for E&M services may not be reported with CPT codes 96372 and 96401.
Reporting Not Otherwise Classified (NOC) Drugs
HCPCS codes J3490 (unclassified drugs) and J3590 (unclassified biological drugs) should be reported for those drugs that have not been assigned a code. In general, claims with these codes must be submitted with additional qualifying information such as the narrative description reflective of the agent (name of the drug and National Drug Code number), the dose administered, and the method of administration. When billing Medicare, report one unit of service in the 2400/SV1-04 data element or in item 24G of the CMS 1500 form. Billing requirements for NOC codes may vary by payer, so providers should consult with the payer to determine coverage and coding requirements.
Frequently Asked Questions
What is the difference between JW and JZ modifiers?
The JW modifier is used in medical billing to indicate the amount of medication that wasn’t given to the patient but was instead wasted or discarded. It’s important to note that the JW modifier should only be used in cases where the amount of wasted medication is significant. On the other hand, the JZ modifier is used to indicate that none of the medication in a single-dose container was wasted or discarded. Read more about the role of modifiers in medical billing by clicking here.
What are some common JW modifier mistakes?
It’s common to feel unsure about ‘which modifier goes where’. Consult a seasoned biller for clarification before submitting your claim.
How can I avoid making mistakes when using a JW modifier?
Create a readily accessible cheat sheet that outlines the meanings of common modifiers. This resource can serve as a quick reference guide to ensure that your team is accurately coding and billing claims, ultimately reducing the risk of claim denials or reimbursement delays.
Reference: https://www.ncbi.nlm.nih.gov/books/NBK569383/
Read more from our published resources:
THE FIVE VITAL STEPS IN GETTING A MEDICAL CLAIM PAID
VIRTUAL SUPERVISION: EXPANDING ACCESS TO HEALTHCARE DURING THE PHE
ABOUT INGA ELLZEY AND OUR DERMATOLOGY BILLING SERVICES
After 28 years of perfecting billing processes, Inga Ellzey continues to be the nation’s leading expert in dermatology billing. Our billing service serves over 100 dermatology practices in 37 states without utilizing any offshore labor. Our goal is to provide our clients and their patients with the most competent and professional service available on the market today.
If you are interested in speaking with our company about how we can maximize your collections while also improving your process, please contact us or call 888-434-4374.

